2019-nCoV IgM/IgG Test (Colloidal Gold)
Product Detail:
Innovita® 2019-nCoV IgM/IgG Test is intended for the qualitative detection of IgM and IgG antibodies against 2019 Novel Coronavirus (2019-nCoV) in human serum/plasma/venous whole blood specimen.
It is used as a supplementary detection indicator for suspected nucleic acid negative results or in conjunction with nucleic acid detection in the diagnosis of suspected cases.
Principle:
The kit detects 2019-nCoV IgM and IgG antibodies by immuno-capture method. The nitrocellulose membrane is coated by mouse-anti human monoclonal IgM ( μ chain) antibodies, mouse-anti human monoclonal IgG ( γ chain) antibodies, and goat-anti- mouse IgG antibodies. The recombinant 2019-nCoV antigen and mouse IgG antibodies are labeled with colloidal gold as a tracer. After addition of the specimens, if 2019-nCoV IgM antibodies are present, the antibodies will bind to colloidal gold-coated 2019-nCoV antigens to form compounds, which are further captured by pre-coated mouse-anti human IgM antibodies to form new compounds, and generate purple or red line (T). If 2019- nCoV IgG antibodies are present in specimen, the antibodies will bind to colloidal gold-labeled 2019-nCoV antigens to form compounds, and further form new compounds by binding to pre-coated mouse-anti human monoclonal IgG ( γ chain) antibodies, which give rise to purple or red line (T). The binding of colloidal gold-labeled mouse IgG antibodies with goat-anti-mouse IgG antibodies will present purple or red line, which is used as the control line (C).
Composition:
|
IFU |
1 |
|
Test cassette |
40 |
|
Specimen diluent |
6mL * 2 bottles |
Test Procedure:
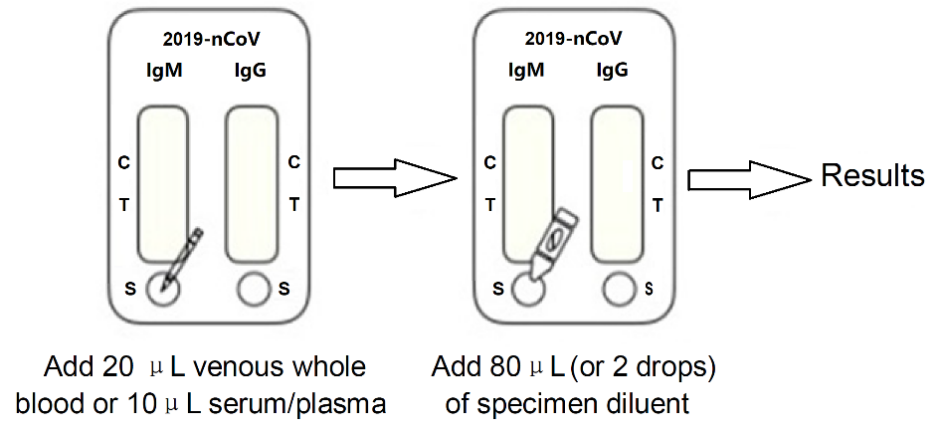
1. Remove the test device from the sealed aluminium foil pouch.
2. Add 20µL venous whole blood or 10µL serum/plasma specimen into each specimen well, and then add 80µL or 2 drops of specimen diluent into each specimen well. Wait for the colored line (s) to appear at room temperature. Read results within 15 minutes.
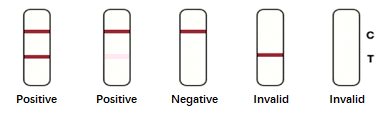
Results Interpretation: