2019-nCoV Neutralizing Antibody Test (Colloidal Gold)
Product Detail:
Innovita® 2019-nCoV IgM/IgG Test is intended for semi-quantitative detection of neutralizing antibodies to the novel coronavirus (2019-nCoV) in human serum, plasma or whole blood specimens.
2019-nCoV includes four main structural proteins: S protein, E protein, M protein and N protein. The RBD region of S protein can bind to the human cell surface receptor ACE2. Neutralizing antibody refers to the ability to bind with the pathogen, and then block the pathogen to invade the body to cause infection. Detection of neutralizing antibody can be used to assess the prognosis of viral infection.
Principle:
The kit is a colloid gold immunochromatography competition assay to detect neutralizing antibodies to 2019-nCoV in human serum, plasma or whole blood specimens. After the specimen is applied to the specimen well, if the neutralizing antibodies are present in the specimen, the neutralizing antibodies will react with the colloidal gold labeled RBD antigen to form the immune complex, and the neutralizing site of labeled RBD antigen would be closed. Then the immune complex and the labeled RBD antigen without binding to neutralizing antibody migrate along the nitrocellulose membrane. When they reach the test zone (T line), the labeled RBD antigen without binding to neutralizing antibodies will react with the ACE2 antigen coated on the nitrocellulose membrane and form a purple-red line. When the concentration of neutralizing antibodies is higher than the lowest detection limit, the purple-red line is lighter than the control line (C line) or there is no purple-red line formed, the result is positive. When the concentration of neutralizing antibodies is lower than the lowest detection limit or there are no neutralizing antibodies in the specimen, the purple-red line is darker than the control line, the result is negative.
Regardless of whether the specimen contains 2019-nCoV neutralizing antibodies, when the colloidal gold-labeled chicken IgY antibody migrates to the control line (C line), it will be captured by the goat anti-chicken IgY antibody precoated on the control line (C line), a purple-red line is formed. The control line (C line) is used as a procedural control. The control lines should always appear in the result windows if the test procedure is performed properly and the reagents are working as intended..
Composition:
|
IFU |
1 |
|
Test cassette |
40 |
|
Specimen diluent |
6mL * 2 bottles |
Test Procedure:
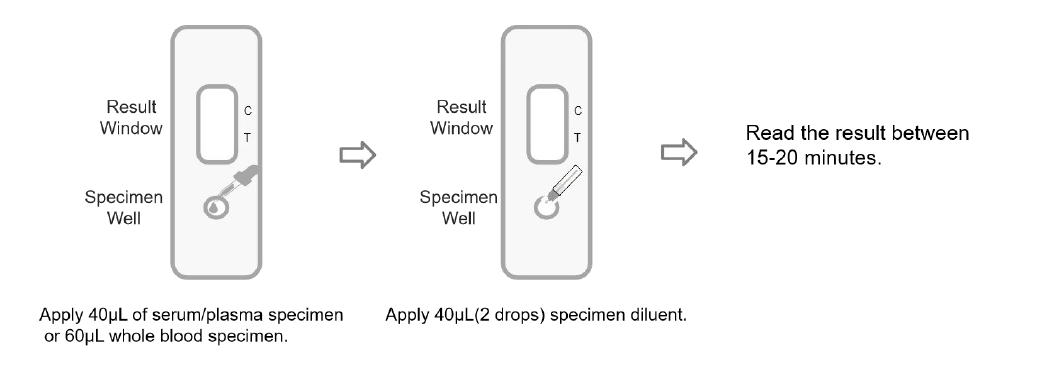
1. Unseal the aluminum foil pouch and take out the test cassette.
2. Apply 40μL of serum/plasma specimen or 60μL whole blood specimen to the specimen well.
3. Apply 40μL (2 drops) specimen diluent to the specimen well.
4. Place it at room temperature (15℃~30℃) for 15-20 minutes, and read the result.
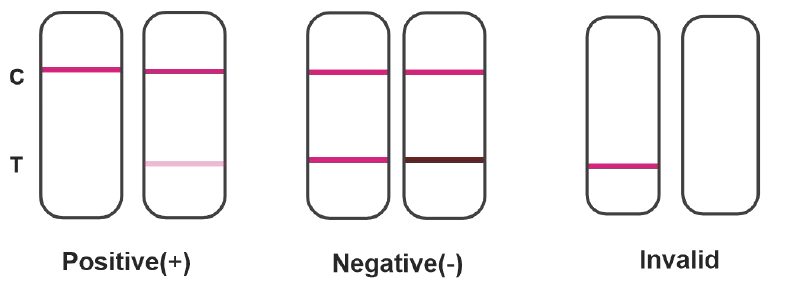
Results Interpretation:
1. Positive: When the color of T line is lighter than that of C line or when there’s no T line, it indicates positive for neutralizing antibodies.
2. Negative: When the color of T line is darker than or equal to that of C line, it indicates negative for neutralizing antibodies.
3. Invalid: When the C line fails to appear, no matter whether the T line is visible or not, the test is invalid. Repeat the test with a new test.