2019-nCoV Neutralizing Antibody Test (QDIC)
Product Detail:
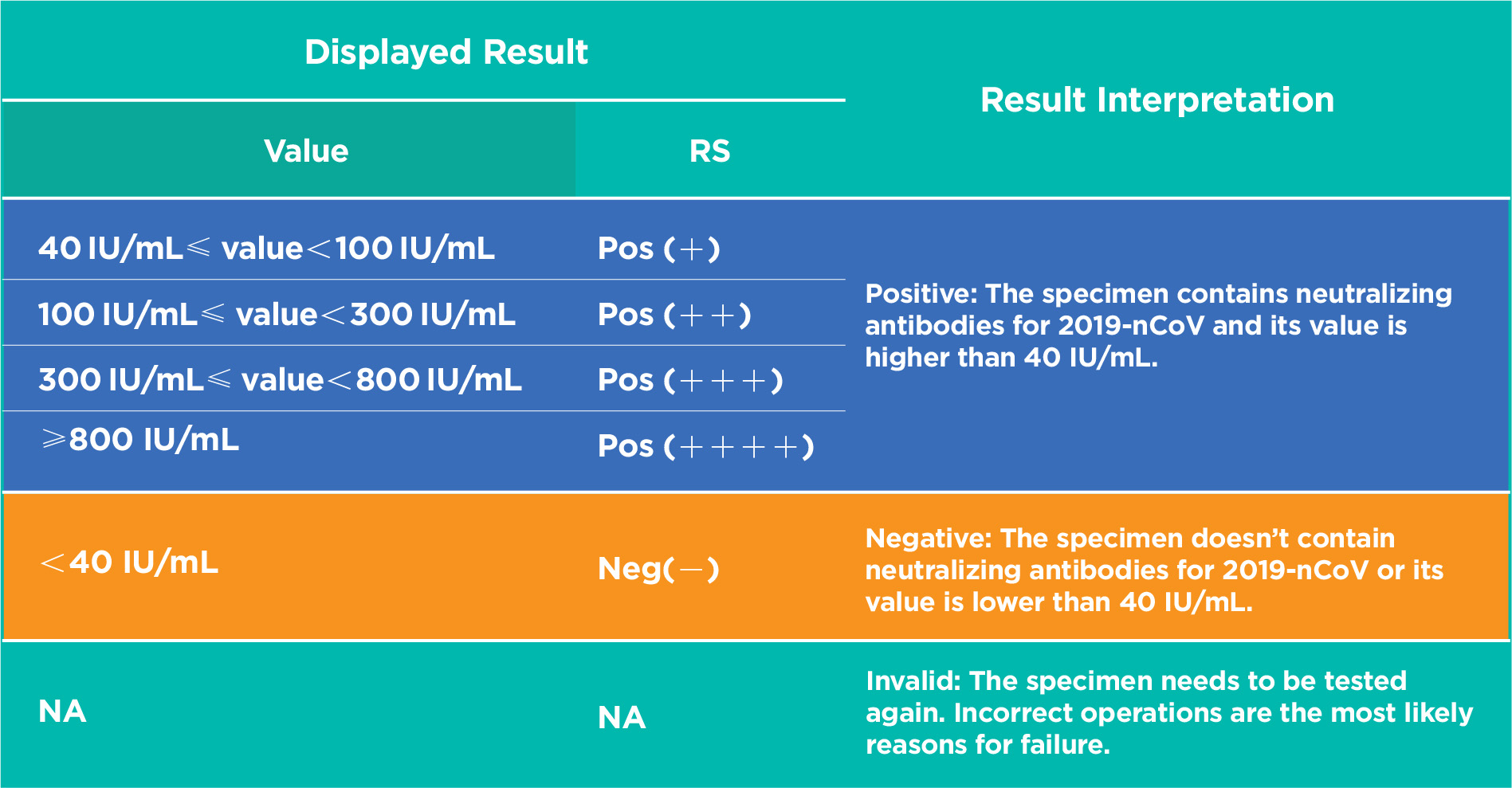
Innovita® 2019-nCoV IgM/IgG Test is intended for quantitative detection of neutralizing antibody to the novel coronavirus (2019-nCoV) in human serum, plasma or whole blood (fingertip blood or venous whole blood) specimens.
2019-nCoV includes four main structural proteins: S protein, E protein, M protein and N protein. The RBD region of S protein can bind to the human cell surface receptor ACE2. Studies have shown that specimens of people who have recovered from the novel coronavirus infection are positive for neutralizing antibody. Detection of neutralizing antibody can be used to assess the prognosis of viral infection and effect evaluation after vaccination.
Principle:
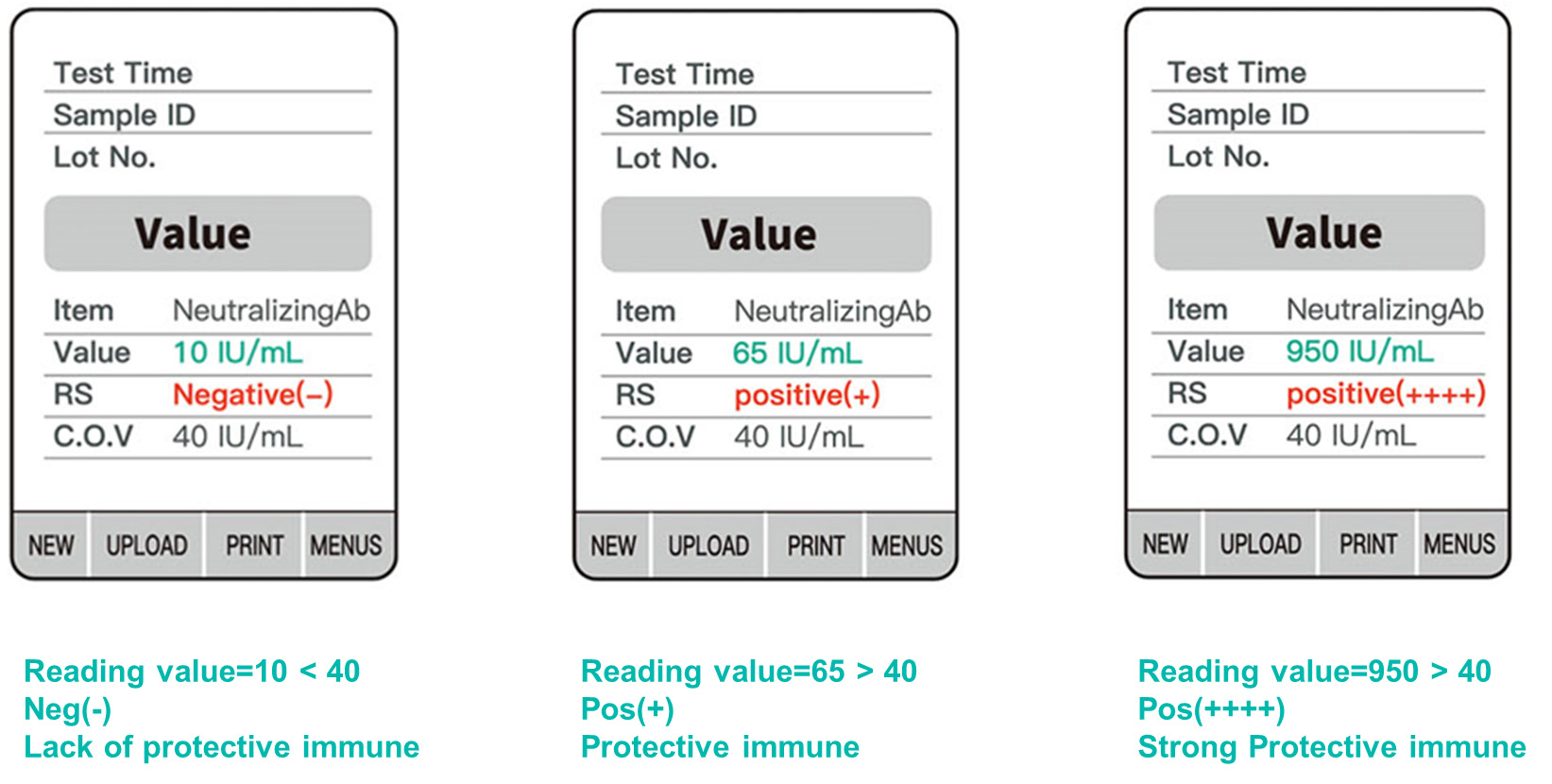
The kit is a quantum dot immunofluorescence chromatography assay to detect 2019-nCoV RBD specific IgG neutralizing antibodies in human serum, plasma or whole blood (fingertip blood and venous whole blood) specimens. After the specimen is applied to the specimen well, if the concentration of neutralizing antibodies is higher than the lowest detection limit, the RBD specific IgG antibodies will react with part or all RBD antigen labeled with the quantum dot microspheres to form the immune compound. Then the immune compound will migrate along the nitrocellulose membrane. When they reach the test zone (T line), the compound will react with the mouse anti-human IgG (γ chain) coated on the nitrocellulose membrane and form a fluorescent line. Read the fluorescence signal value with the fluorescence immunoassay analyzer. The signal value is proportional to the content of neutralizing antibodies in the specimen.
Whether the specimen contains RBD specific neutralizing antibodies or not, the control line should always appear in the result window if the test procedure is performed properly and the reagent is working as intended. When the chicken IgY antibody labeled with quantum dot microspheres migrates to the control line (C line), it will be captured by the goat anti-chicken IgY antibody precoated on the C line, and a fluorescent line is formed. The control line (C line) is used as a procedural control.

Composition:
|
Composition |
Amount |
Specification |
|
IFU |
1 |
/ |
|
Test cassette |
20 |
Each sealed foil pouch containing one test device and one desiccant |
|
Specimen diluent |
3mL*1 vial |
20mM PBS, Sodium Casein, ProClin 300 |
|
Micropipette |
20 |
Micropipette with 20μL marker line |
|
Lancet |
20 |
/ |
|
Alcohol pad |
20 |
/ |
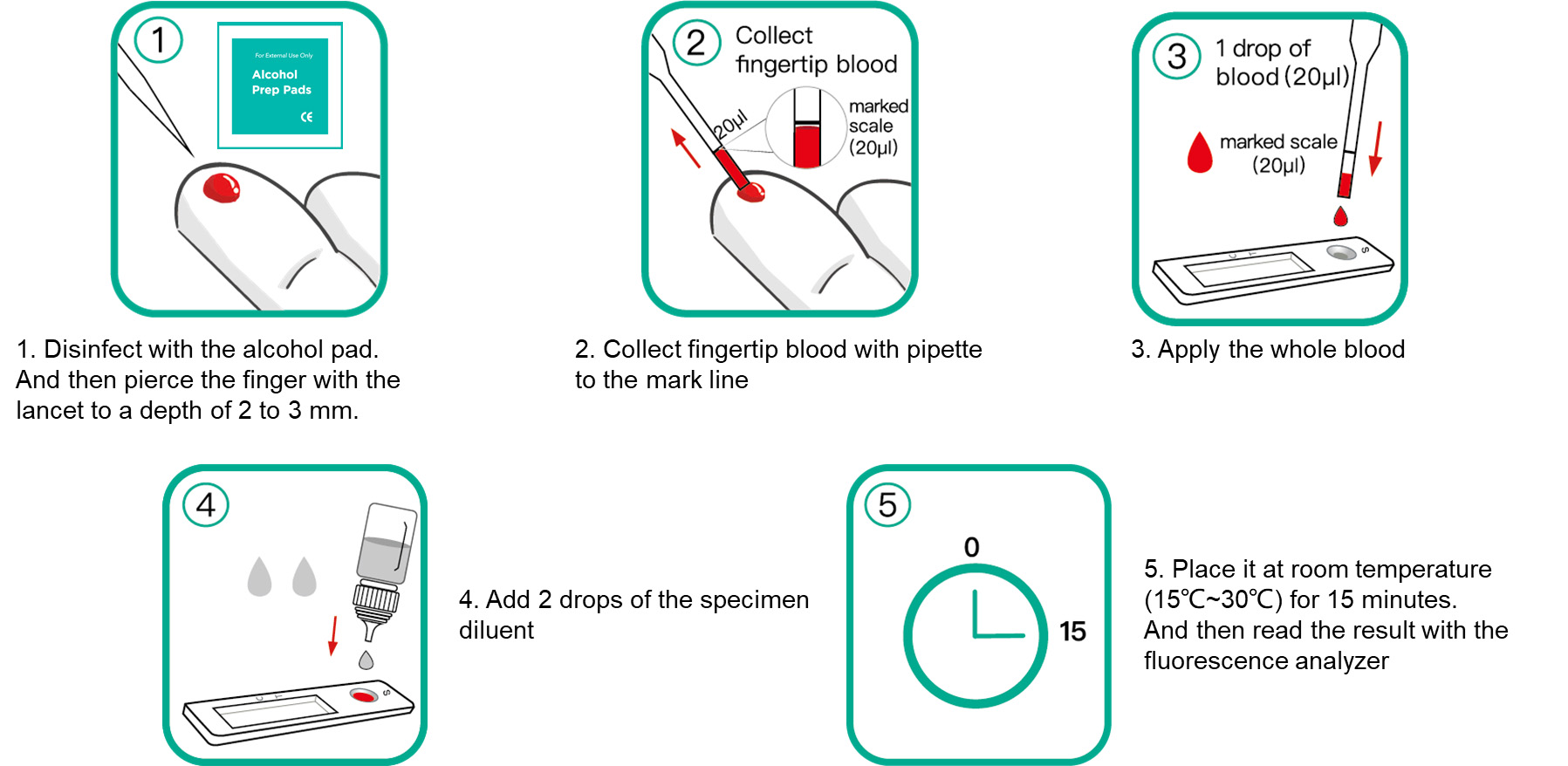
Test Procedure:
● Fingertip Blood Collection
● Read the result with the fluorescence analyzer