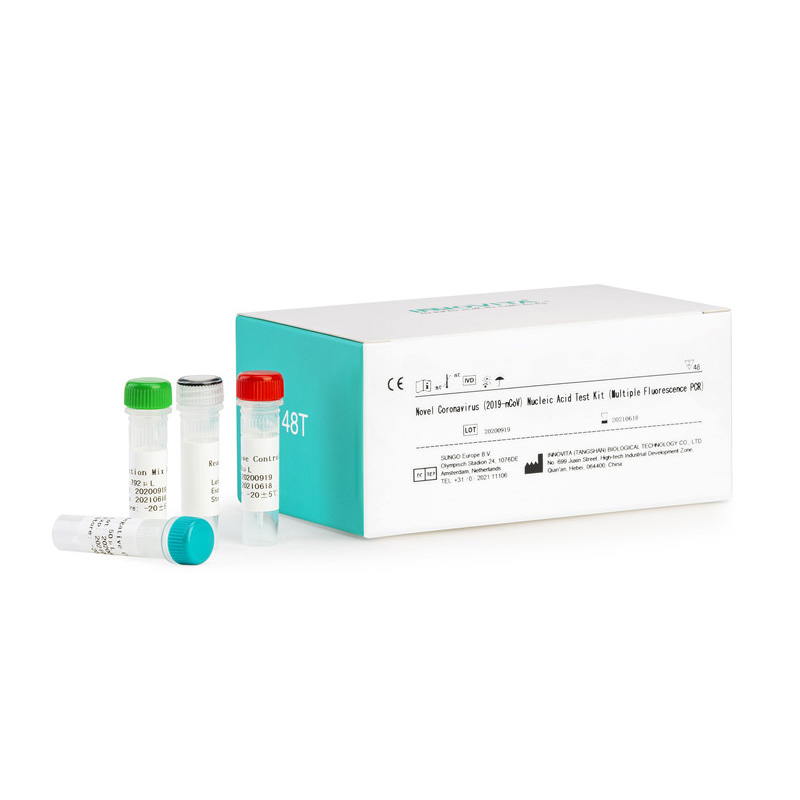
CE Certification Igg/Igm Rapid Test Kit Factory Quotes – Novel Coronavirus (2019-nCoV) Nucleic Acid Test Kit (Multiple Fluorescence PCR) – INNOVITA
CE Certification Igg/Igm Rapid Test Kit Factory Quotes – Novel Coronavirus (2019-nCoV) Nucleic Acid Test Kit (Multiple Fluorescence PCR) – INNOVITA Detail:
Product Detail:
Innovita® 2019-nCoV IgM/IgG Test is intended for the diagnosis and epidemiological monitoring of diseases caused by the novel coronavirus (2019-nCoV). ORF1ab and N gene of 2019-nCoV are detected qualitatively from throat swabs and alveolar lavage fluid samples collected from suspected cases of pneumonia, patients with suspected cases, others who need to diagnose.
The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive analysis of the condition based on the patient’s clinical manifestations and other laboratory tests.
Principle:
This kit uses one-step reverse transcription polymerase chain reaction (RT-PCR) detection technology to target the novel coronavirus (2019-nCoV) ORF1ab gene, N gene, and human internal reference gene sequence. Specific primers and taqman probes were designed in conserved regions.
Composition:
|
Composition |
48 tests / kit |
| Reaction Mix A | 792μL×1Tube |
| Reaction Mix B | 168μL×1Tube |
| Positive Control | 50μL×1Tube |
| Negative Control | 50μL ×1Tube |
Note: 1. Different batches of reagents should not be mixed.
2. Positive controls and positive controls need not be extracted
Test Procedure:
1. Nucleic acid extraction:
Commercial RNA extraction kits are available, magnetic bead extraction and spin column extraction are recommended for this kit.
2. Preparation the Reaction Mix:
● Take out the 2019-nCoV reaction Mix A/B and keep at room temperature until unfreezing;
● Take the corresponding portions (Reaction Mix A 16.5μL/T, Reaction Mix B 3.5μL/T) and mix, and then aliquot each PCR reaction with 20μL/ tube;
● Add 5μL of RNA template or negative control or positive control, then cover the tube cap;
● Place the reaction tube in the fluorescence PCR instrument, and set the negative / positive control and sample parameters for RT-PCR reaction according to the instrument operating instructions.
● Record Sample placement order
3.RT-PCR protocols:
Recommended Settings:
|
Cycle |
Time |
Temperature(℃) |
|
|
1 |
1 |
10min |
25 |
|
2 |
1 |
10min |
50 |
|
3 |
1 |
10min |
95 |
|
4 |
45 |
10s |
95 |
|
35s |
55 |
Product detail pictures:
Related Product Guide:
We have now a hugely efficient team to deal with inquiries from buyers. Our goal is "100% client gratification by our solution high-quality, rate & our team service" and take pleasure in a great popularity among clients. With several factories, we will provide a wide assortment of CE Certification Igg/Igm Rapid Test Kit Factory Quotes – Novel Coronavirus (2019-nCoV) Nucleic Acid Test Kit (Multiple Fluorescence PCR) – INNOVITA , The product will supply to all over the world, such as: Liberia, Iran, Malta, Qualified R&D engineer will be there for your consultation service and we will try our best to meet your requirements. So please feel free to contact us for inquiries. You'll be able to send us emails or call us for small business. Also you are able to come to our business by yourself to get further knowing of us. And we will surely give you the best quotation and after-sale service. We're ready to build stable and friendly relations with our merchants. To achieve mutual success, we will make our best efforts to build a solid co-operation and transparent communication work with our companions. Above all, we are here to welcome your inquiries for any of our goods and service.
The enterprise has a strong capital and competitive power, product is sufficient, reliable, so we have no worries on cooperating with them.