CE Certification Parainfluenza Virus Type 1 Factory Quotes – 2019-nCoV Ag Test (Latex Chromatography Assay) / Professional Test / Saliva – INNOVITA
CE Certification Parainfluenza Virus Type 1 Factory Quotes – 2019-nCoV Ag Test (Latex Chromatography Assay) / Professional Test / Saliva – INNOVITA Detail:
Product Detail:
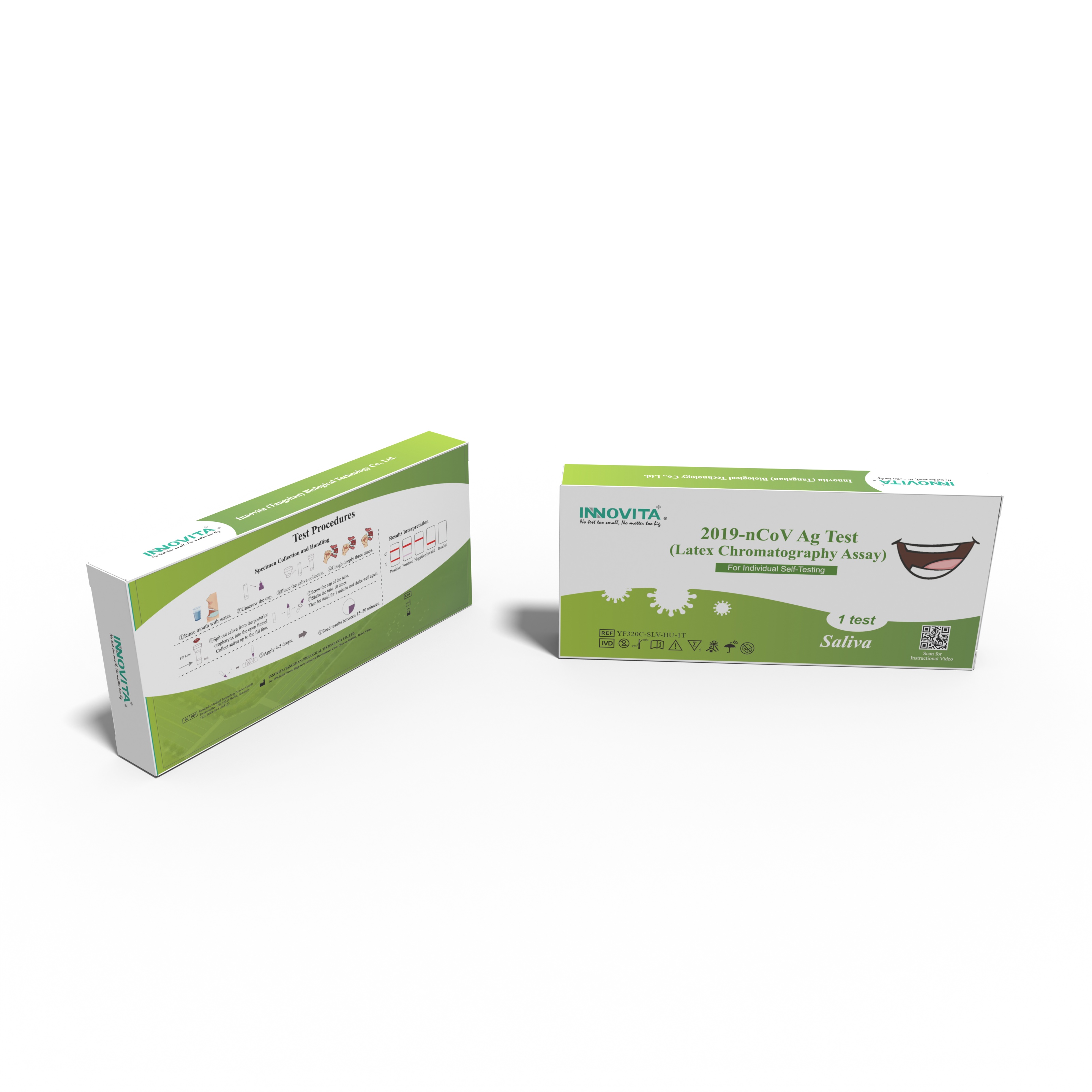
Innovita® 2019-nCoV Ag Test is intended for the direct and qualitative detection of SARS-CoV-2 nucleocapsid protein antigen in saliva from individuals who are suspected of COVID-19 by their healthcare provider within the first seven days of the onset of symptoms or for screening of individuals without symptoms or other reasons to suspect COVID-19 infection.
The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive analysis of the condition based on the patient’s clinical manifestations and other laboratory tests.
Principle:
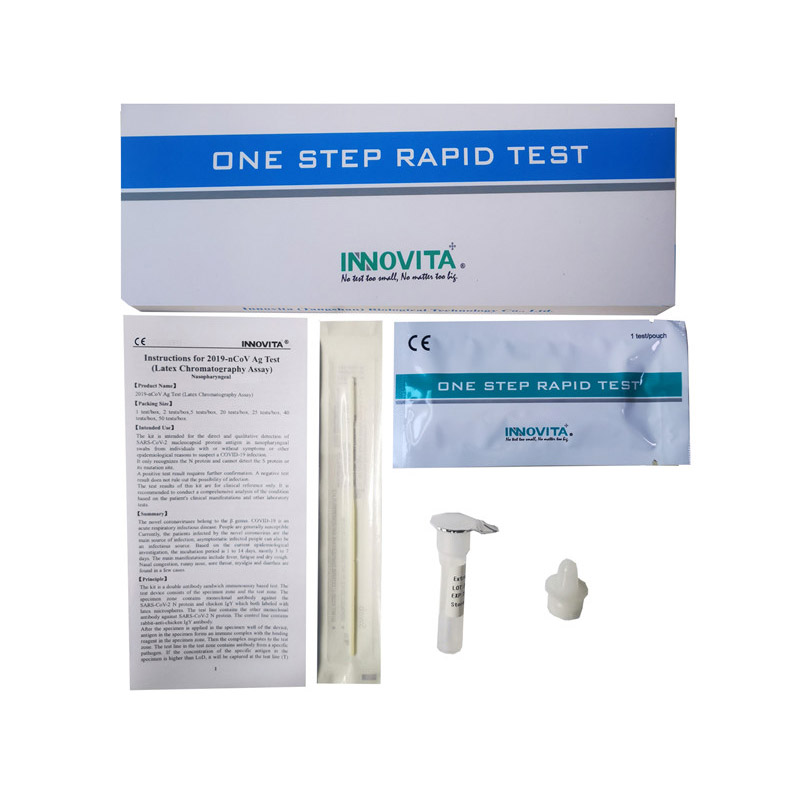
The kit is a double antibody sandwich immunoassay based test. The test device consists of the specimen zone and the test zone. The specimen zone contains monoclonal antibody against the SARS-CoV-2 N protein and chicken IgY which both labeled with latex microspheres. The test line contains the other monoclonal antibody against SARS-CoV-2 N protein. The control line contains rabbit-anti-chicken IgY antibody.
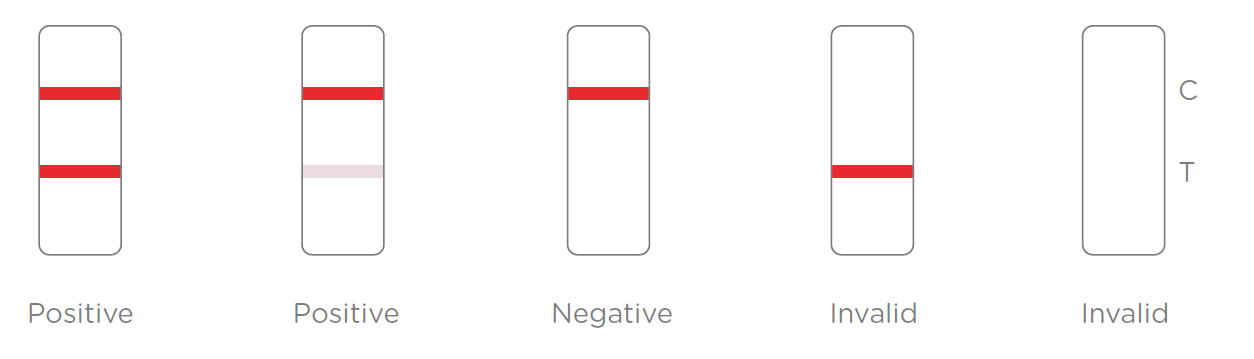
After the specimen is applied in the specimen well of the device, antigen in the specimen forms an immune complex with the binding reagent in the specimen zone. Then the complex migrates to the test zone. The test line in the test zone contains antibody from a specific pathogen. If the concentration of the specific antigen in the specimen is higher than LoD, it will be captured at the test line (T) and form a red line. In contrast, if the concentration of the specific antigen is lower than LoD, it will not form a red line. The test also contains an internal control system. A red control line (C) should always appear after the test is completed. Absence of a red control line indicates an invalid result.
Composition:
|
Composition |
Amount |
|
IFU |
1 |
|
Test cassette |
1/20 |
|
Extraction diluent |
1/20 |
|
Saliva collector |
1/20 |
Test Procedure:
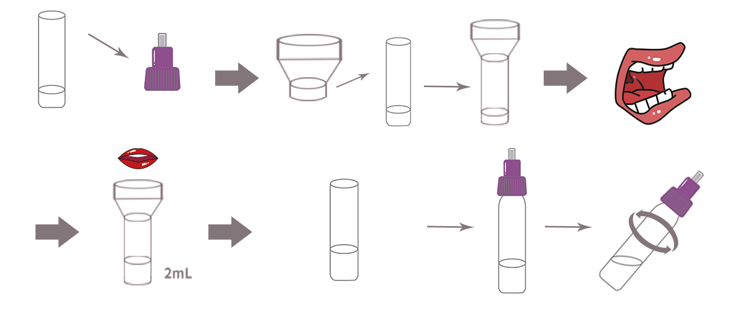
1.Specimen Collection and Handling
● Unscrew the cap of the extraction diluent and place the saliva collector on it.
● Rinse mouth with water. Cough deeply three times. Spit out saliva from the posterior oropharynx into the open funnel. Collect saliva through the saliva collector up to the fill line. Do not exceed the fill line.
● Remove the saliva collector and screw the lid of the sample tube back on.
● Shake the test tube 10 times so that the saliva mixes thoroughly with the extraction diluent. Then let stand for 1 minute and shake well again.
* If the saliva sample is visibly cloudy, leave it to settle before testing.Specimen
2.Test Procedure
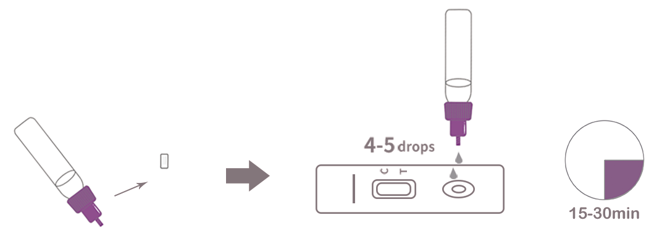
● Allow test device, specimen and diluent equilibrate to room temperature 15~30℃ prior to opening the pouch. Remove the test device from the sealed aluminum foil pouch.
● Apply 4-5 drops of the test specimen into the specimen well.
● Wait for the red line(s) to appear at room temperature. Read results between 15~30 minutes. Do not read the result after 30 minutes.
Results Interpretation:
Product detail pictures:

Related Product Guide:
Our crew through skilled training. Skilled skilled knowledge, strong sense of company, to meet the company wants of customers for CE Certification Parainfluenza Virus Type 1 Factory Quotes – 2019-nCoV Ag Test (Latex Chromatography Assay) / Professional Test / Saliva – INNOVITA , The product will supply to all over the world, such as: Bangkok, Amman, Bulgaria, Since always, we adhering to the "open and fair, share to get, the pursuit of excellence, and creation of value"values, adhere to the"integrity and efficient, trade-oriented, best way , best valve" business philosophy. Together with our all over the world have branches and partners to develop new business areas, maximum common values. We sincerely welcome and together we share in global resources, opening up new career together with the chapter.
The goods are very perfect and the company sales manager is warmful, we will come to this company to purchase next time.