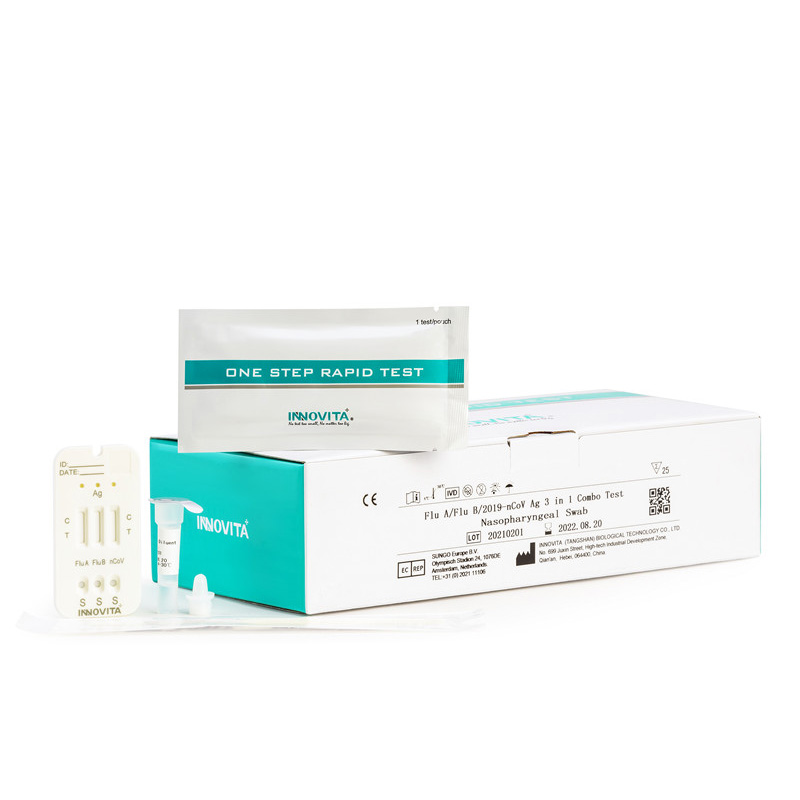
CE Certification PCR test Manufacturers Exporters – 2019-nCoV Ag Test (Latex Chromatography Assay) / Professional Test / Nasopharyngeal Swab – INNOVITA
CE Certification PCR test Manufacturers Exporters – 2019-nCoV Ag Test (Latex Chromatography Assay) / Professional Test / Nasopharyngeal Swab – INNOVITA Detail:
Product Detail:
Innovita® 2019-nCoV Ag Test is intended for the direct and qualitative detection of SARS-CoV-2 nucleocapsid protein antigen in nasopharyngeal swabs from individuals who are suspected of COVID-19 by their healthcare provider within the first seven days of the onset of symptoms or for screening of individuals without symptoms or other reasons to suspect COVID-19 infection.
The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive analysis of the condition based on the patient’s clinical manifestations and other laboratory tests.
Principle:
The kit is a double antibody sandwich immunoassay based test. The test device consists of the specimen zone and the test zone. The specimen zone contains monoclonal antibody against the SARS-CoV-2 N protein and chicken IgY which both labeled with latex microspheres. The test line contains the other monoclonal antibody against SARS-CoV-2 N protein. The control line contains rabbit-anti-chicken IgY antibody.
After the specimen is applied in the specimen well of the device, antigen in the specimen forms an immune complex with the binding reagent in the specimen zone. Then the complex migrates to the test zone. The test line in the test zone contains antibody from a specific pathogen. If the concentration of the specific antigen in the specimen is higher than LoD, it will be captured at the test line (T) and form a red line. In contrast, if the concentration of the specific antigen is lower than LoD, it will not form a red line. The test also contains an internal control system. A red control line (C) should always appear after the test is completed. Absence of a red control line indicates an invalid result.
Composition:
|
Composition |
Amount |
|
IFU |
1 |
|
Test cassette |
1/25 |
|
Extraction diluent |
1/25 |
|
Dropper tip |
1/25 |
|
Swab |
1/25 |
Test Procedure:
1.Specimen Collection
Place the swab into one of the patient’s nostrils until it reaches the posterior nasopharynx; keep inserting until resistance is encountered or the distance is equivalent to that from the ear to the nostril of the patient. The swab should be rotated on the nasopharyngeal mucosa for 5 times or more, and then taken out.
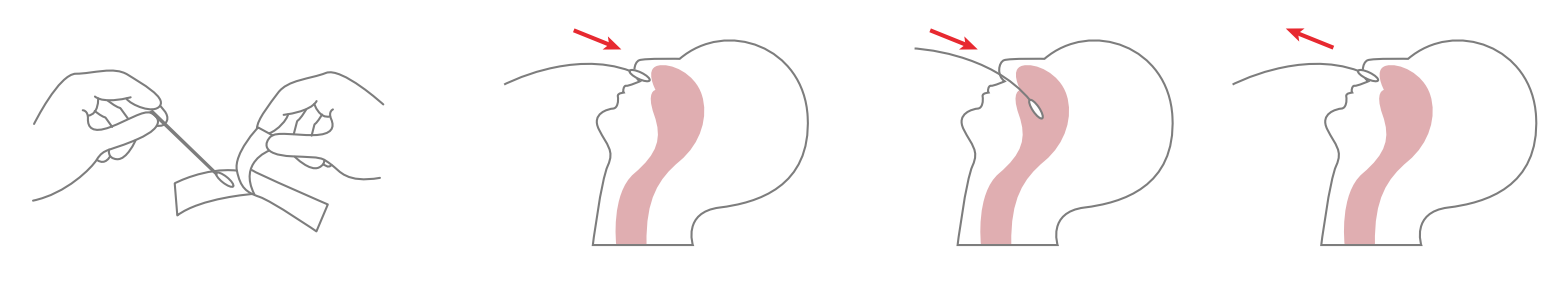
2.Specimen Handling
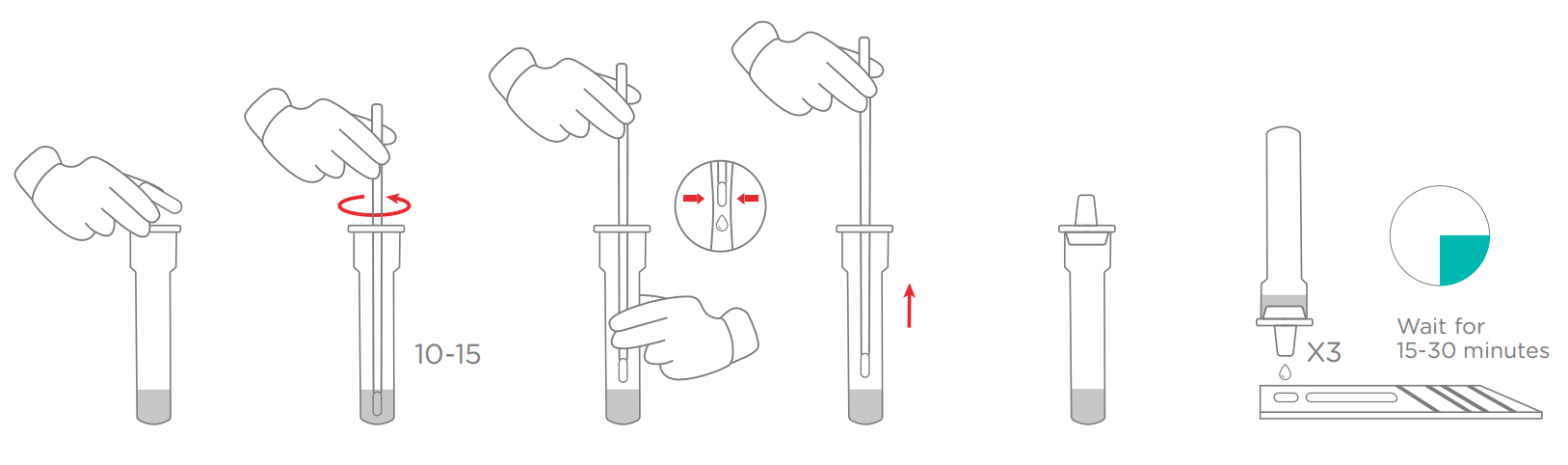
3.Test Procedure

● Allow test device, specimen and diluent to equilibrate to room temperature 15~30℃ prior to opening the pouch. Remove the test device from the sealed aluminum foil pouch.
● Apply 3 drops of the test specimen into the specimen well.
● Wait for the red line(s) to appear at room temperature. Read results between 15~30 minutes. Do not read the result after 30 minutes.
Results Interpretation:
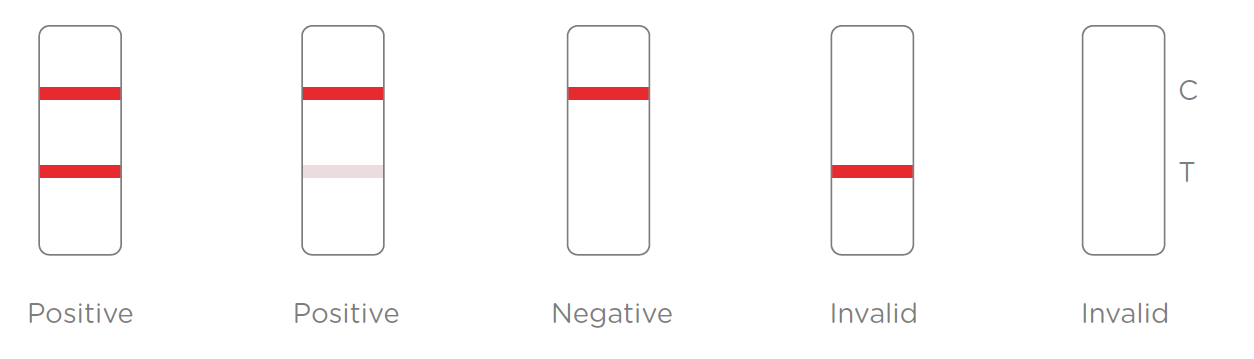
Product detail pictures:

Related Product Guide:
Our eternal pursuits are the attitude of "regard the market, regard the custom, regard the science" and the theory of "quality the basic, trust the first and management the advanced" for CE Certification PCR test Manufacturers Exporters – 2019-nCoV Ag Test (Latex Chromatography Assay) / Professional Test / Nasopharyngeal Swab – INNOVITA , The product will supply to all over the world, such as: Tajikistan, Argentina, Pretoria, We honor ourselves as a company that comprises of a strong team of professionals who are innovative and well experienced in the international trading, business development and product advancement. Moreover, the company stays unique among its competitors due to its superior standard of quality in production, and its efficiency and flexibility in business support.
The product manager is a very hot and professional person, we have a pleasant conversation, and finally we reached a consensus agreement.