CE Certification Rotavirus Antigen Factories Pricelist – Flu A/Flu B/2019-nCoV Ag 3 in 1 Combo Test – INNOVITA
CE Certification Rotavirus Antigen Factories Pricelist – Flu A/Flu B/2019-nCoV Ag 3 in 1 Combo Test – INNOVITA Detail:
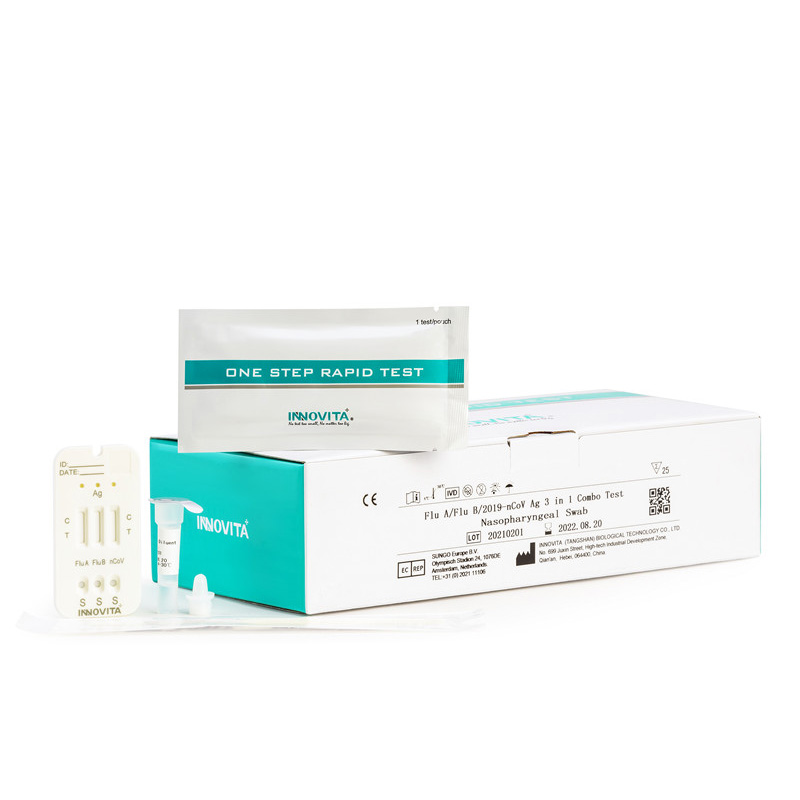
Product Detail:
Innovita® Flu A/Flu B/2019-nCoV Ag 3 in 1 Combo Test is intended for the qualitative detection and differentiation of nucleocapsid antigen from Influenza Virus type A, Influenza Virus type B and 2019-nCoV directly from nasopharyngeal swab specimens obtained from individuals.
It can be only used in professional institutions.
A positive test result requires further confirmation. A negative test result does not rule out the possibility of infection.
The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive analysis of the condition based on the patient’s clinical manifestations and other laboratory tests.
Principle:
The kit is a double antibody sandwich immunoassay-based test. The test device consists of the specimen zone and the test zone.
1) Flu A/Flu B Ag: The specimen zone contains monoclonal antibody against the Flu A/Flu B N protein. The test line contains the other monoclonal antibody against Flu A/Flu B protein. The control line contains goat-anti-mouse IgG antibody.
2) 2019-nCoV Ag: The specimen zone contains monoclonal antibody against the 2019-nCoV N protein and chicken IgY. The test line contains the other monoclonal antibody against 2019-nCoV N protein. The control line contains rabbit-anti-chicken IgY antibody.
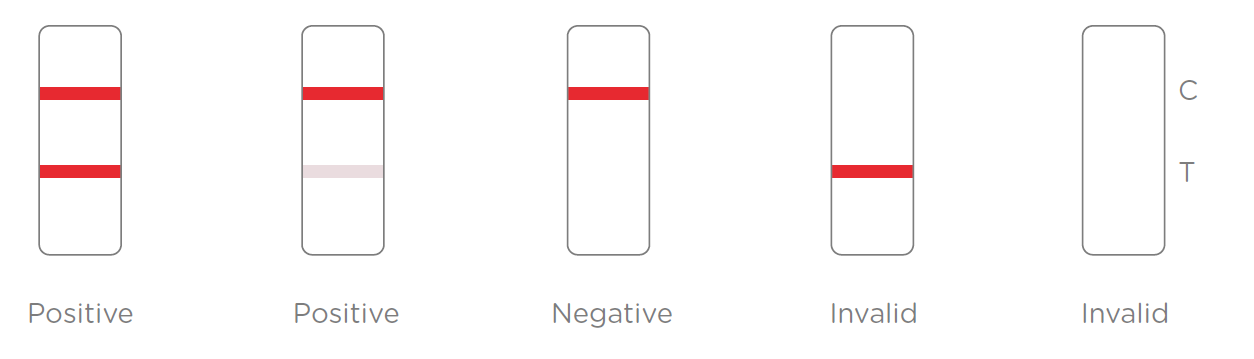
After the specimen is applied in the specimen well of the device, antigen in the specimen forms an immune complex with the binding antibody in the specimen zone. Then the complex migrates to the test zone. The test line in the test zone contains antibody from a specific pathogen. If the concentration of the specific antigen in the specimen is higher than LOD, it will form a purple-red line at the test line (T). In contrast, if the concentration of the specific antigen is lower than LOD, it will not form a purple-red line. The test also contains an internal control system. A purple-red control line (C) should always appear after the test is completed. Absence of a purple-red control line indicates an invalid result.
Composition:
|
Composition |
Amount |
Specification |
|
IFU |
1 |
/ |
|
Test cassette |
25 |
Each sealed foil pouch containing one test device and one desiccant |
|
Extraction diluent |
500μL*1 Tube *25 |
Tris-Cl buffer, NaCl, NP 40, ProClin 300 |
|
Dropper tip |
25 |
/ |
|
Swab |
25 |
/ |
Test Procedure:
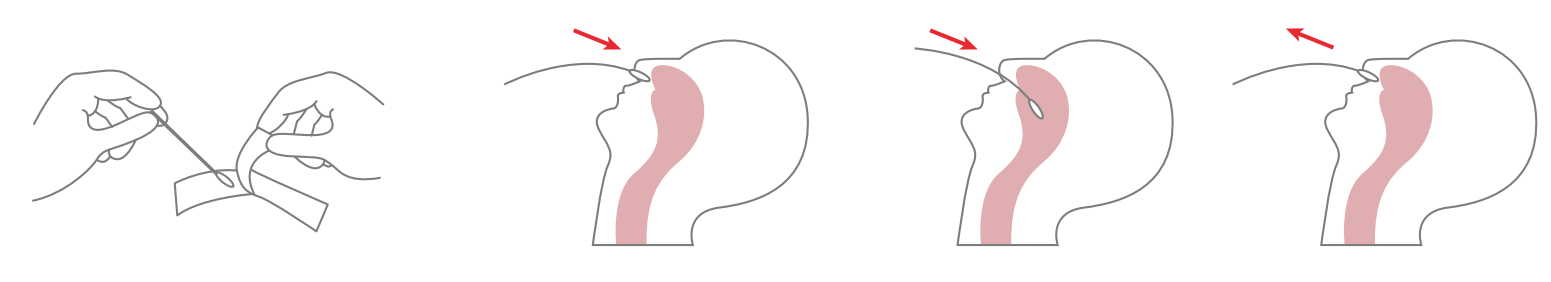
1. Specimen collection requirements:
1.Place the swab into one of the patient’s nostrils until it reaches the posterior nasopharynx; keep inserting until resistance is encountered or the distance is equivalent to that from the ear to the nostril of the patient. The swab should be rotated on the nasopharyngeal mucosa for 5 times or more, and then taken out.
2.Freshly collected dry swabs should be processed as soon as possible, but no later than 1 hour after specimen collection.
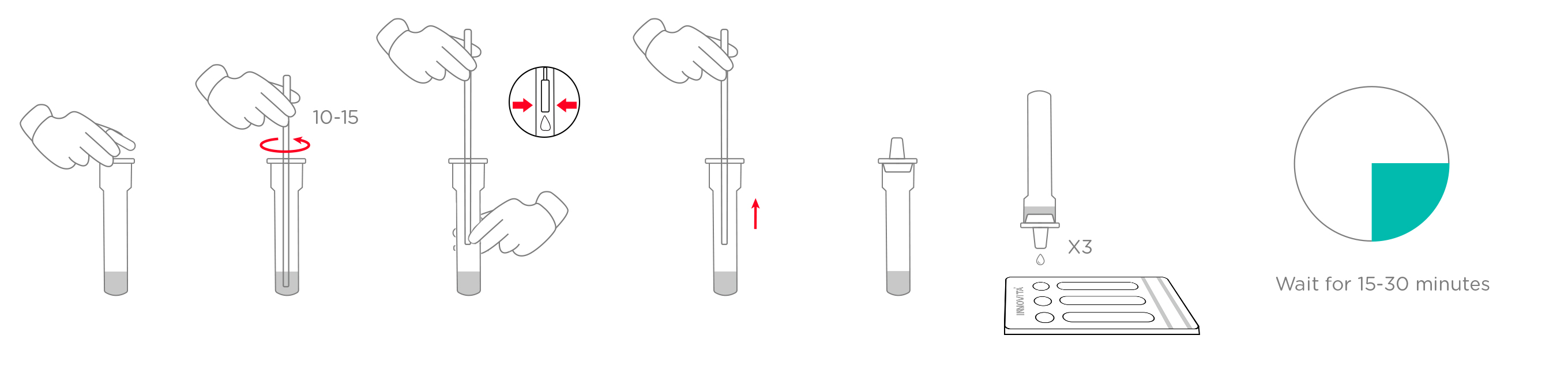
2. Specimen handling:
3.Results Interpretation
Product detail pictures:
Related Product Guide:
continue to enhance, to be certain solution top quality in line with market and buyer standard requirements. Our corporation has a excellent assurance program are actually established for CE Certification Rotavirus Antigen Factories Pricelist – Flu A/Flu B/2019-nCoV Ag 3 in 1 Combo Test – INNOVITA , The product will supply to all over the world, such as: Armenia, Finland, Provence, Our company insists on the principle of "Quality First, Sustainable Development", and takes "Honest Business, Mutual Benefits" as our developable goal. All members sincerely thank all old and new customers' support. We will keep working hard and offering you the highest-quality products and service.
Factory equipment is advanced in the industry and the product is fine workmanship, moreover the price is very cheap, value for money!