-
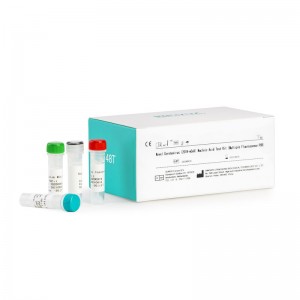
CE Certification One Step HCG Pregnancy Urine Test Kit Factories Pricelist – Novel Coronavirus (2019-nCoV) Nucleic Acid Test Kit (Multiple Fluorescence PCR) – INNOVITA
Product Detail: Innovita® 2019-nCoV IgM/IgG Test is intended for the diagnosis and epidemiological monitoring of diseases caused by the novel coronavirus (2019-nCoV). ORF1ab and N gene of 2019-nCoV are detected qualitatively from throat swabs and alveolar lavage fluid samples collected from suspected cases of pneumonia, patients with suspected cases, others who need to diagnose. The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive analysis... -
High-Quality OEM CP Factories Pricelist – 2019-nCoV Ag Test (Latex Chromatography Assay) / Professional Test / Saliva – INNOVITA
Product Detail: Innovita® 2019-nCoV Ag Test is intended for the direct and qualitative detection of SARS-CoV-2 nucleocapsid protein antigen in saliva from individuals who are suspected of COVID-19 by their healthcare provider within the first seven days of the onset of symptoms or for screening of individuals without symptoms or other reasons to suspect COVID-19 infection. The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive analysis of th... -
High-Quality OEM IgM Manufacturers Exporters – Flu A/Flu B/2019-nCoV Ag 3 in 1 Combo Test – INNOVITA
Product Detail: Innovita® Flu A/Flu B/2019-nCoV Ag 3 in 1 Combo Test is intended for the qualitative detection and differentiation of nucleocapsid antigen from Influenza Virus type A, Influenza Virus type B and 2019-nCoV directly from nasopharyngeal swab specimens obtained from individuals. It can be only used in professional institutions. A positive test result requires further confirmation. A negative test result does not rule out the possibility of infection. The test results of this kit a... -

High-Quality OEM Drug test Urine Kit Manufacturers Exporters – Novel Coronavirus (2019-nCoV) Nucleic Acid Test Kit (Multiple Fluorescence PCR) – INNOVITA
Product Detail: Innovita® 2019-nCoV IgM/IgG Test is intended for the diagnosis and epidemiological monitoring of diseases caused by the novel coronavirus (2019-nCoV). ORF1ab and N gene of 2019-nCoV are detected qualitatively from throat swabs and alveolar lavage fluid samples collected from suspected cases of pneumonia, patients with suspected cases, others who need to diagnose. The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive analysis... -
CE Certification Testing Kit Manufacturers Exporters – 2019-nCoV Ag Test (Latex Chromatography Assay) / Professional Test / Anterior Nasal Swab – INNOVITA
Product Detail: Innovita® 2019-nCoV Ag Test is intended for the direct and qualitative detection of SARS-CoV-2 nucleocapsid protein antigen in anterior nasal swabs from individuals who are suspected of COVID-19 by their healthcare provider within the first seven days of the onset of symptoms or for screening of individuals without symptoms or other reasons to suspect COVID-19 infection. The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive ... -

High-Quality OEM IFA Manufacturers Exporters – Novel Coronavirus (2019-nCoV) Nucleic Acid Test Kit (Multiple Fluorescence PCR) – INNOVITA
Product Detail: Innovita® 2019-nCoV IgM/IgG Test is intended for the diagnosis and epidemiological monitoring of diseases caused by the novel coronavirus (2019-nCoV). ORF1ab and N gene of 2019-nCoV are detected qualitatively from throat swabs and alveolar lavage fluid samples collected from suspected cases of pneumonia, patients with suspected cases, others who need to diagnose. The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive analysis... -

High-Quality OEM Drug test Factories Pricelist – Novel Coronavirus (2019-nCoV) Nucleic Acid Test Kit (Multiple Fluorescence PCR) – INNOVITA
Product Detail: Innovita® 2019-nCoV IgM/IgG Test is intended for the diagnosis and epidemiological monitoring of diseases caused by the novel coronavirus (2019-nCoV). ORF1ab and N gene of 2019-nCoV are detected qualitatively from throat swabs and alveolar lavage fluid samples collected from suspected cases of pneumonia, patients with suspected cases, others who need to diagnose. The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive analysis... -

High-Quality OEM Fertility test Factory Quotes – 2019-nCoV Ag Test (Latex Chromatography Assay) / Professional Test / Saliva – INNOVITA
Product Detail: Innovita® 2019-nCoV Ag Test is intended for the direct and qualitative detection of SARS-CoV-2 nucleocapsid protein antigen in saliva from individuals who are suspected of COVID-19 by their healthcare provider within the first seven days of the onset of symptoms or for screening of individuals without symptoms or other reasons to suspect COVID-19 infection. The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive analysis of th... -
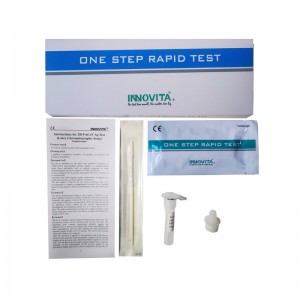
CE Certification MP Factory Quotes – 2019-nCoV Ag Test (Latex Chromatography Assay) / Professional Test / Nasopharyngeal Swab – INNOVITA
Product Detail: Innovita® 2019-nCoV Ag Test is intended for the direct and qualitative detection of SARS-CoV-2 nucleocapsid protein antigen in nasopharyngeal swabs from individuals who are suspected of COVID-19 by their healthcare provider within the first seven days of the onset of symptoms or for screening of individuals without symptoms or other reasons to suspect COVID-19 infection. The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive ... -

High-Quality OEM point-of-care testing Factory Quotes – Flu A/Flu B/2019-nCoV Ag 3 in 1 Combo Test – INNOVITA
Product Detail: Innovita® Flu A/Flu B/2019-nCoV Ag 3 in 1 Combo Test is intended for the qualitative detection and differentiation of nucleocapsid antigen from Influenza Virus type A, Influenza Virus type B and 2019-nCoV directly from nasopharyngeal swab specimens obtained from individuals. It can be only used in professional institutions. A positive test result requires further confirmation. A negative test result does not rule out the possibility of infection. The test results of this kit a... -

CE Certification China CE Virus Home Test Kit Antigen Rapid Test Kits Factories Pricelist – 2019-nCoV Ag Test (Latex Chromatography Assay) / Professional Test / Anterior Nasal Swab – INNOVITA
Product Detail: Innovita® 2019-nCoV Ag Test is intended for the direct and qualitative detection of SARS-CoV-2 nucleocapsid protein antigen in anterior nasal swabs from individuals who are suspected of COVID-19 by their healthcare provider within the first seven days of the onset of symptoms or for screening of individuals without symptoms or other reasons to suspect COVID-19 infection. The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive ... -
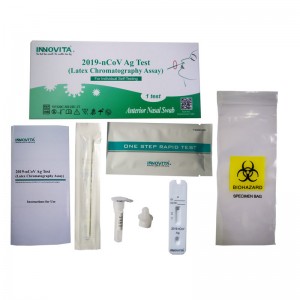
High-Quality OEM TORCH Manufacturers Exporters – 2019-nCoV Ag Test (Latex Chromatography Assay) / Self-Test / Anterior Nasal Swab – INNOVITA
Product Detail: Innovita® 2019-nCoV Ag Test is intended for the direct and qualitative detection of SARS-CoV-2 nucleocapsid protein antigen in anterior nasal swab that is self-collected by an individual aged 18 years or older or is collected by an adult from young individuals. It only recognizes the N protein and cannot detect the S protein or its mutation site. The kit is intended for layperson as self-testing at home or at work (in offices, for sports events, airports, schools, etc.) What ...







